Last Updated on March 20, 2024 by
Alternative to Meds Editorial Team
Medically Reviewed by Dr John Motl MD
Last Updated on March 20, 2024 by
Alternative to Meds Editorial Team
Medically Reviewed by Dr John Motl MD
WARNING: The FDA advises against abrupt Depakote withdrawal except where a life-threatening condition requires immediate cessation.2 Always get medical advice and guidance before gradually stopping an antiepileptic, anticonvulsive drug.
Medical literature on Depakote withdrawal (divalproex sodium, valproic acid) is extremely limited. Depakote also comes in a delayed-release version, extended-release form, tablet form, a syrup formulation, and “sprinkles” designed for being added to soft food. We have attempted to collate as much information as possible below, and we caution that medical oversight is a must for coming off any anticonvulsive or antiseizure medication, regardless of the reasons it may have been prescribed.2,9,13,15
Other changes reported when stopping Depakote included:
*Depakote is FDA-approved to be prescribed for bipolar episodes of mania. According to clinical studies, bipolar conditions are often misdiagnosed where other contributing factors are at play and are not investigated thoroughly.3,4
Physicians often prescribe mood stabilizers and anticonvulsants, like Depakote, as a lifetime treatment for a fixed diagnosis. But is this really the best treatment overall?
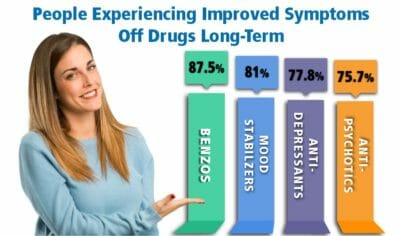
We have provided information below for anyone looking for information about Depakote withdrawal symptoms, divalproex sodium side effects, and holistic treatment help for Depakote withdrawal. Depakote comes in 5 different oral forms, given here in descending order of absorption rates:
VPA syrup: (valproic acid) sold as “Depakene”
VPA capsule: (valproic acid)
Divalproex sodium sprinkles Depakote sprinkles come in a capsule that can be opened and added to soft food
Divalproex sodium enteric-coated – delayed-release tablet
Divalproex sodium ER – extended-release tablet
You will find more information below regarding drug classification, information about its mechanism of action, long-term effects, and other treatment information including holistic treatment for Depakote withdrawal. Depakote is an FDA-approved anti-seizure (antiepileptic) medication.2
Black box warnings: include fatal liver disease, major birth defects such as spina bifida and neural tube malformations, and rapidly emerging life-threatening pancreatitis in all age groups. Though not included in the black box, a nearly doubled risk compared to placebo for suicidal thinking and behavior has been demonstrated in clinical trials in ALL populations.2,16
Depakote is used in the treatment of seizure disorders, such as epilepsy, often in combination with other medications.
The FDA approved Depakote for the treatment of bipolar disorder after an unspecified number of 3-week long trials.2 Bipolar disorder has a high propensity for misdiagnosis.3,4 Manic episodes might be considered a bipolar symptom and are described as distinct periods of abnormally sustained elevated mood and other characteristics including irritability and hostility. Other mania symptoms include machine gun-type speech, reduced need to sleep, grandiosity, absence of good judgment, motor hyperactivity, and what can appear to be an expansive flood of fanciful ideas. Depakote was not safety-tested for longer 3-weeks for mania and cautions closely monitoring and reevaluating the usefulness of Depakote for a patient in this situation.2

Please note: Depakote is not recommended for pregnant women or women of child-bearing age for any reason due to the risks to what is now termed “fetal valproate syndrome.” Children born to mothers taking valproate during pregnancy may suffer withdrawals at birth, and there is a high risk for the baby to be born with birth defects or other medical problems. These adverse events were documented in clinical trials authored by Thisted and Ebbeson, and as reported by the National Organization of Rare Diseases.2,5,19,20
Depakote is sometimes used as an adjunctive or add-on medication in the management of schizophrenia, psychosis, or other psychiatric disorders. particular caution is advised when other medications are prescribed as they may slow or speed up the rate at which Depakote is metabolized.
Off-label uses include the treatment of alcohol or other drug dependencies, diabetic neuropathy, and to quell impulsivity and aggressive behavior such as after traumatic brain injury, based on rat studies showing decreased permeability of the blood-brain barrier in rats who were given impacts to the brain with an electromagnetic cortical-impact device followed by valproate, and another study linking valproate with a decrease in neuronal damage after inducing continuous and repeated electrically-induced seizures in rats.16,17,18 Where a drug does not have an accessible and robust body of documentation concerning human long-term clinical trials to rely on, one would be well-advised to do one’s own research on such medication as carefully and as thoroughly as possible to make informed decisions about starting or stopping such drugs. Additional important information on these topics follows.
It is vital to note that Depakote (valproate) was approved for treating bipolar disorder after only very short trials were done on hospitalized acute mania patients. No long-term trials were ever completed that demonstrated the long-term safety or effectiveness of Depakote. Nonetheless, Depakote has been prescribed to millions of people since its approval in 1983. There is a much larger body of evidence that has accumulated post-marketing of the drug that includes adverse side effects, symptoms of withdrawal, and other information below.
Always seek reliable medical advice before starting or stopping a prescription drug such as Depakote, and before adding other medications that may interact and cause unexpected reactions.

The FDA label indicates that where hepatic dysfunction, pancreatitis, toxic levels of ammonia or other life-threatening conditions emerge, Depakote should be immediately stopped.2
If tapering is done correctly it can be a mild procedure. Done too fast, it can be extremely difficult and can introduce undesirable risks to health.
Other trade names for the generic valproate, sodium valproate, or Divalproex delayed-release, include Depakene, Depakote ER (extended-release), Depakote Sprinkles, Stavzor, and Alti-Valproic.
The drug is available in a soft-gel capsule, tablet, or syrup form. Depakote Sprinkles are designed so that if a person cannot swallow a capsule or tablet, the drug can be sprinkled on applesauce or other types of soft food. One capsule of Sprinkles is meant to be used all at once.

The FDA2 has published some known adverse side effects that require careful monitoring while on Depakote, including:
Below, some information is provided regarding the most frequently asked questions about Depakote, concerning the mechanism of action, how it can affect the body adversely, and other things to be aware of if you are considering Depakote withdrawal.

As a fundamental aspect of Depakote withdrawal, we also want to investigate the root causes for the symptoms that led to prescription medication in the first place. Headache sufferers who have been prescribed heavy drugs like Depakote may be interested in research published on how certain nutrient deficiencies are directly linked to migraine and other types of chronic debilitating headaches. For example, in a study published in the Journal of Head and Face Pain, Mauskop et al found 80% of their trial participants experienced headache relief within 15 minutes of intravenous magnesium sulfate.21 In their book “Magnesium in Headache,” authors Yablon et al further demonstrate the benefits of magnesium in treating migraine headaches in this free book published by the Adelaide University Press.14
The health goals of the individual are of primary importance at our center. Using such protocols as holistic neurotransmitter replacement therapy, toxicity removal, testing for and correcting nutritional deficits, IV treatments, and many other helpful holistic techniques, we can help a person regain optimal health, while at the same reducing the need for prescription drugs.
Each person has uniqueness in their make-up, genetic profile, sensitivities, strengths, and personal goals. That is why each client at the center receives a treatment plan tailored to meet those goals.
More information is available on request, and we would welcome further inquiries for more details on our therapies and treatments for Depakote withdrawal that Alternative to Meds Center could provide you for your bridge back to the best levels of health with the least amount of prescription medication possible.
1. Verrotti A, D’Egidio C, Mohn A, Coppola G, Chiarelli F. Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev. 2011 May;12(5):e32-43. doi: 10.1111/j.1467-789X.2010.00800.x. Epub 2010 Sep 6. PMID: 20880119. [cited 2022 Aug 1]
2. FDA label Depakote (divalproex sodium) 1983 [cited 2022 Aug 1]
3. Shen H, et al., “Analysis of Misdiagnosis of Bipolar Disorder in an Outpatient Setting.” Shanghai Arch Psychiatry April 25 2018 PMID 29736129 [cited 2022 Aug 1]
4. Bowden CL. “Strategies to reduce misdiagnosis of bipolar depression.” Psychiatr Serv. 2001 Jan;52(1):51-5. doi: 10.1176/appi.ps.52.1.51. PMID: 11141528.[cited 2022 Aug 1]
5. Osterweil N, “Depakote Has High Birth Defects Rate Among Antiepileptics.” MedpageToday [online} August 8 2006 [cited 2022 Aug 1]
6. Schwartz TL, Massa JL, Gupta S, Al-Samarrai S, Devitt P, Masand PS. Divalproex Sodium Versus Valproic Acid in Hospital Treatment of Psychotic Disorders. Prim Care Companion J Clin Psychiatry. 2000;2(2):45-48. doi:10.4088/pcc.v02n0203 [cited 2022 Aug 1]
7. Sherr JD, Kelly DL. Substitution of immediate-release valproic acid for divalproex sodium for adult psychiatric inpatients. Psychiatr Serv. 1998 Oct;49(10):1355-7. doi: 10.1176/ps.49.10.1355. PMID: 9779912.[cited 2022 Aug 1]
8. Rahman M, Nguyen H. Valproic Acid. [Updated 2021 Jan 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559112/ [cited 2022 Aug 1]
9. Wylie T, Sandhu DS, Murr N. Status Epilepticus. 2021 May 19. In: StatPearls Treasure Island (FL): StatPearls Publishing; 2021 Jan–. PMID: 28613459. [cited 2022 Aug 1]
10. Lossius MI, Hessen E, Mowinckel P, Stavem K, Erikssen J, Gulbrandsen P, Gjerstad L. Consequences of antiepileptic drug withdrawal: a randomized, double-blind study (Akershus Study). Epilepsia. 2008 Mar;49(3):455-63. doi: 10.1111/j.1528-1167.2007.01323.x. Epub 2007 Sep 19. PMID: 17888074.[cited 2022 Aug 1]
11. Jacoby A, Johnson A, Chadwick D. Psychosocial outcomes of antiepileptic drug discontinuation. The Medical Research Council Antiepileptic Drug Withdrawal Study Group. Epilepsia. 1992 Nov-Dec;33(6):1123-31. doi: 10.1111/j.1528-1157.1992.tb01769.x. PMID: 1464274. [cited 2022 Aug 1]
12. Ranganathan LN, Ramaratnam S. Rapid versus slow withdrawal of antiepileptic drugs. Cochrane Database Syst Rev. 2006 Apr 19;(2):CD005003. doi: 10.1002/14651858.CD005003.pub2. PMID: 16625621. [cited 2022 Aug 1]
13. Pavese N, Baracchini G, Bonuccelli U. Valproate-withdrawal induced migraine. Headache. 1994 Jul-Aug;34(7):445. doi: 10.1111/j.1526-4610.1994.hed3407445_1.x. PMID: 7928333. [cited 2022 Aug 1]
14. Yablon LA, Mauskop A. Magnesium in headache. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System [Internet]. Adelaide (AU): University of Adelaide Press; 2011. PMID: 29920023.[cited 2022 Aug 1]
15. Duncan JS, Shorvon SD, Trimble MR. Withdrawal symptoms from phenytoin, carbamazepine and sodium valproate. J Neurol Neurosurg Psychiatry. 1988 Jul;51(7):924-8. doi: 10.1136/jnnp.51.7.924. PMID: 3144581; PMCID: PMC1033195. [cited 2022 Aug 1]
16. NAMI information sheet, “Valproate (Depakote)” June 2019 [online] [cited 2022 Aug 1]
17. Dash PK, Orsi SA, Zhang M, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One. 2010;5(6):e11383. Published 2010 Jun 30. doi:10.1371/journal.pone.0011383 [cited 2022 Aug 1]
18. Brandt C, Gastens AM, Sun Mz, Hausknecht M, Löscher W. Treatment with valproate after status epilepticus: effect on neuronal damage, epileptogenesis, and behavioral alterations in rats. Neuropharmacology. 2006 Sep;51(4):789-804. doi: 10.1016/j.neuropharm.2006.05.021. Epub 2006 Jun 27. PMID: 16806297. [cited 2022 Aug 1]
19. NORD authors, “Fetal Valproate Syndrome” [online] 2020 [cited 2022 Aug 1]
20. Thisted E, Ebbesen F. Malformations, withdrawal manifestations, and hypoglycaemia after exposure to valproate in utero. Arch Dis Child. 1993 Sep;69(3 Spec No):288-91. doi: 10.1136/adc.69.3_spec_no.288. PMID: 8215567; PMCID: PMC1029494. [cited 2022 Aug 1]
21. Mauskop A, Altura B, Cracco R, Altura BM, “Intravenous Magnesium Sulfate Rapidly Alleviates Headaches of Various Types” Headache Journal of Head and Face Pain March 1998 [online] [cited 2022 Aug 1]
22. Rahman M, Nguyen H. Valproic Acid. [Updated 2021 Jan 26]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK559112/ [cited 2022 Aug 1]
23. National Center for Biotechnology Information (2021). PubChem Compound Summary for CID 3121, Valproic acid. Retrieved July 15, 2021 from https://pubchem.ncbi.nlm.nih.gov/compound/Valproic-acid. [cited 2022 Aug 1]
24. Patel AR, Nagalli S. Valproate Toxicity. [Updated 2021 Apr 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560898/ [[cited 2022 Aug 1]
Originally Published Sep 13, 2018 by Diane Ridaeus

Dr. Motl is currently certified by the American Board of Psychiatry and Neurology in Psychiatry, and Board eligible in Neurology and licensed in the state of Arizona. He holds a Bachelor of Science degree with a major in biology and minors in chemistry and philosophy. He graduated from Creighton University School of Medicine with a Doctor of Medicine. Dr. Motl has studied Medical Acupuncture at the Colorado School of Traditional Chinese Medicine and at U.C.L.A.

Diane is an avid supporter and researcher of natural mental health strategies. Diane received her medical writing and science communication certification through Stanford University and has published over 3 million words on the topics of holistic health, addiction, recovery, and alternative medicine. She has proudly worked with the Alternative to Meds Center since its inception and is grateful for the opportunity to help the founding members develop this world-class center that has helped so many thousands regain natural mental health.
Can you imagine being free from medications, addictive drugs, and alcohol? This is our goal and we are proving it is possible every day!
Read All StoriesView All Videos